technology
rooted in cutting-edge scienceOUR APPROACH “REMUSCULARITION”
We selectively produce a large amount of ventricular type cardiomyocytes from iPS cells. Our original purification method (patented in major countries), which eliminates undifferentiated cells and non-cardiomyocytes, minimizes the risk of teratoma formation and arrhythmia.
The transplanted differentiated cardiomyocytes are efficiently engrafted in the host myocardium for a long period of time, and is expected to contribute to the long-term improvement of cardiac function of patients with heart failure. This is called “Remuscularization".
Among the many technologies related to cardiac regenerative medicine, the transplant method of differentiated cardiomyocytes is an extremely important factor that determines the therapeutic effect. Our direct injection method of differentiated cardiomyocytes has been reported that the differentiated cardiomyocytes transplanted directly into the myocardium can electrically couple with the recipient's cardiomyocytes, and good results have been demonstrated in various animal experiments.
Original technologies in each step with a view of industrialization
iPSC Production
In the conventional iPS cell production method, skin tissue of the patient is collected and reprogramming genes are directly inserted into the genome. However, the possibility of tumorigenicity could not be denied with this method because the genome itself could be damaged. In our method, we can generate iPSCs without direct insertion of reprogramming genes into the genome by using a special vector, and we have succeeded in significantly reducing the possibility of tumorigenicity. In addition, since iPS cells can be generated from only one drop of blood, the burden on patients can be reduced.
iPS cells have self-renewal ability and pluripotency similar to ES cells, but there is a difference in differentiation ability and quality depending on the cell line compared with ES cells, so it is important to secure the quality of iPS cells. We focused on H1 foo, a linker histone protein specifically expressed in oocytes, and we have succeeded in generating iPSCs with enhanced efficiency of embryoid bodies and chimera formation by adding this gene during iPS cell reprogramming.
Differentiation
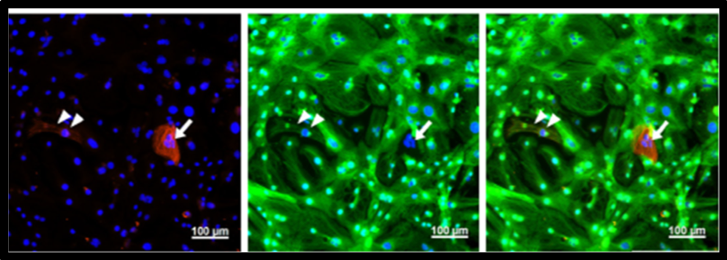
immunofluorescence staining for myosin light chain 2a (MLC2a) and myosin light chain 2v (MLC2v) was performed. Most purified CMs were MLC2v-positive ventricular CM(Tohyama S, et al. Cell Metabolism, 2016)
A large number of cardiomyocytes are required to cardiac regenerative medicine, and several steps are required to differentiate undifferentiated iPS cells into cardiomyocytes. From the many research results of the developmental study of the heart including ours, with related to the induction and differentiation of cardiomyocyte from pluripotent stem cells, it has been found that combining proteins such as BMP/Activin signal and Wnt signal that are involved in early cardiac development can significantly increase the efficiency of differentiation. In addition, research on replacing recombinant proteins with chemical compounds has progressed, and it has become possible to produce cardiomyocytes more inexpensively and efficiently.
On the other hand, not only the signal but also the culture environment is important for efficient cardiomyocyte differentiation. We have already succeeded in developing a clinically applicable level of cardiomyocyte differentiation culture solution through collaboration with companies, and high cardiomyocyte differentiation efficiency can be achieved by combining with a low molecular compounds-based differentiation induction method. In addition, the cardiomyocytes that we produce are ventricular specific ones that work for the pumping function of the heart.
Large-scale cell culture
Although a method has been reported for producing a large number of differentiated cells in a mass by three-dimensional culture, this method has several problems. First, it is difficult to completely remove undifferentiated stem cells that cause tumorigenesis when the cell mass increases. In addition, there are other problems such as the reduction of cell proliferation efficiency, the inability to control the size of cell mass uniformly, and the instability of cardiomyocyte differentiation efficiency.
We have established a two-dimensional mass culture system using multi-layered culture plates. In the case of using a multi-layered culture plate, it was an issue that the proliferation of human iPS cells becomes unstable because the layers cannot be cultured uniformly. By using the forced ventilation system, it has become possible to stabilize the proliferation of human iPS cells and to obtain a sufficiently large number of differentiated cardiomyocytes for transplantation into patients.
Purification
It has been revealed that undifferentiated iPS cells remain after induction of differentiation into cardiomyocytes, and when these undifferentiated cells are mixed in when transplanted into a patient's body, they form teratoma, which is a major problem. Therefore, before transplanting cardiomyocytes, it is necessary to remove undifferentiated cells that cause tumorigenesis and purify only cardiomyocytes.
In the past, the mainstream method was to separate cells one by one using FACS cell sorter and collect only cardiomyocytes. By analyzing energy metabolism of iPS cells and cardiomyocytes in detail, we have succeeded in developing culture solutions, removing undifferentiated cells inexpensively and easily, and recovering a large number of cardiomyocytes. This technological breakthrough has dramatically increased the possibility of iPS cell-derived cardiomyocyte transplantation.
Spheroid
When transplanting purified cardiomyocytes to heart tissue, it has been confirmed that only a small number of cells can be finally engrafted when transplanting disjointed cardiomyocytes one by one. We have been searching for alternative method that can achieve high engraftment rate.
As a result, we have developed a method for efficient transplantation by creating "cardiomyocyte spheroid", in which about 1,000 cardiomyocytes are aggregated, and transplanting them into the heart tissue. Using this method, it has been confirmed that cardiomyocytes made from iPS cells engraft in heart tissue with a high rate, and cardiomyocytes grow further after engraftment and significantly improve actual cardiac function. Our company name, Heartseed, is named after transplanting this "cardiomyocyte spheroid", which will be the heart's seed (Heartseed), to a site that has become necrotic due to myocardial infarction and the like.
In addition, we are developing special transplantation devices for transplantation of cardiomyocyte spheroid and establishing a cell transplantation system that does not rely on the skill of the operator. Development of such a system to reduce the technical burden of such a transplant operator and to transplant cardiomyocyte spheroid in an efficient manner is expected to play an important role in the stability of the therapeutic effect after cell transplantation and the spread of this therapy.
